Amine dehydrogenase enzyme technology
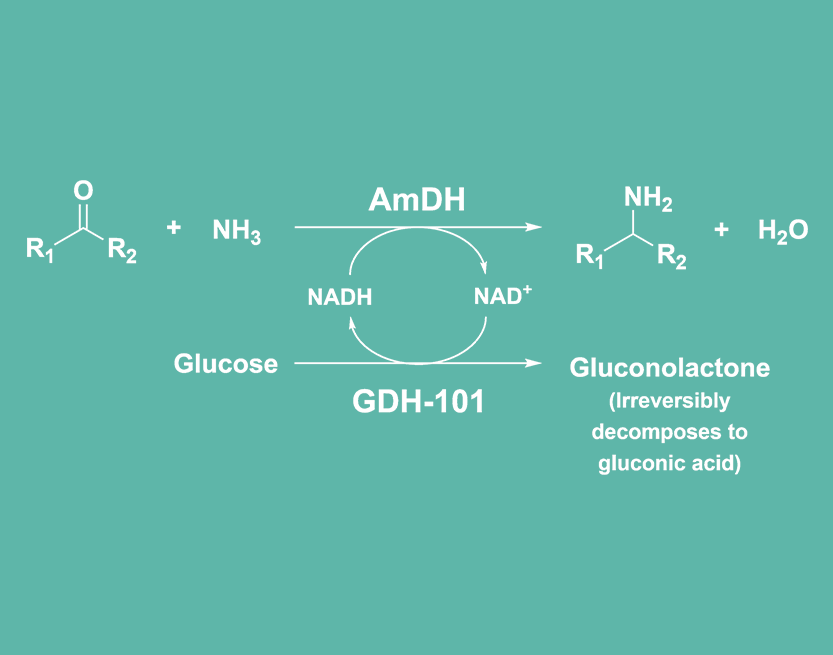
Johnson Matthey’s amine dehydrogenase (AmDH) enzymes are wild type and engineered enzymes stemming from amino acid dehydrogenases, where two or more mutations in the active site have enabled them to catalyse a wider range of transformations (1). Ketones with aromatic moieties can be transformed into the corresponding amine by amine dehydrogenase.
The best enzyme for the transformation of choice might vary depending on the length of the aliphatic chain between the ketone and the aromatic group. Small aliphatic ketones are substrates compatible with AmDH enzymes.
Advantages of using amine dehydrogenase:
- Exhibit high chemo-, regio- and enantioselectivity, producing chiral amines with high enantiomeric excess in aqueous media, and under mild conditions of temperature and pressure.
- AmDH enzymes use ammonia as the nitrogen source, offering simplicity and high efficiency while reducing waste generation.
- Tuneable activity and selectivity by using enzyme engineering.
AmDH enzyme options
Get in touch
Send us a message to request a quote or ask our advice. We'd be happy to hear from you.
Reference:
1. ACS Catal., 2017, 7, 5, 3204-3209
1. ACS Catal., 2017, 7, 5, 3204-3209


