Palladium, although having the lowest melting point and being the least dense of all the PGMs, finds its way into many crucial applications. Whilst mainly used in catalytic converters, it is also an important catalyst for chemical markets. When combined with silver, palladium alloys are also used in medical, military and aerospace applications. In plating applications, it is often alloyed with nickel and gold to offer an excellent combination of conductivity, corrosion resistance and hardness.
Palladium uses
Demand in the palladium market is dominated by its extensive use in automotive emissions control, specifically emissions control used in gasoline vehicles. Platinum is also heavily used in catalytic conversion emission control, but palladium’s higher durability and lower price in recent years made it more favorable. For this reason, catalytic converters often contain a mix of platinum, palladium and rhodium.
Palladium is used in the electronics industry, predominantly as a plating component for printed circuit boards (PCB’s), semiconductor lead frames and connectors. It is also implemented as a paste product for some electronic components such as resistors, capacitors, thermistors and actuators.
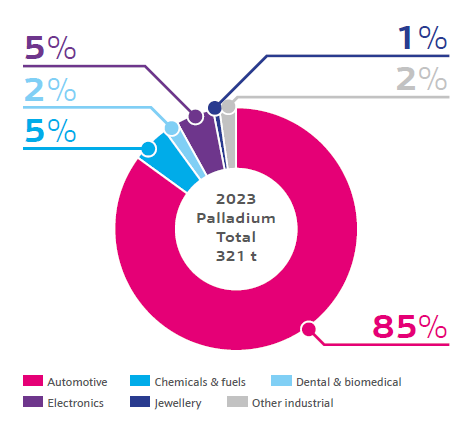

As a catalyst, palladium plays a vital role in producing a variety of bulk and specialty chemicals. These include several key intermediates for plastics manufacturing, hydrogen peroxide, nitric acid, and a huge range of active pharmaceutical compounds. Palladium catalysts are used in these processes for their optimal activity, stability, and selectivity by eliminating unwanted by-products and fully using raw starting materials.
Other uses for palladium include dental alloys, where its good solubility with other metals, biocompatibility and tarnish/corrosion resistance properties make it suitable. Palladium is also used as an alloying component in white gold and platinum jewellery.
Palladium as a catalyst
Read more