Crystallisation process development
21 October 2020
Providing a first-choice opportunity - adopting crystallisation development at an early stage.
In the manufacture of active pharmaceutical ingredients (APIs), crystallisation is a key unit operation. The importance of designing the solid state and particle form of a given API is well-appreciated in the industry as it can impact the downstream processability of the isolated material and can be used to maximise the efficacy of the final drug.
Crystallisation provides the first-choice opportunity to achieve this designed particle, however, historically time and cost pressures have inhibited developing the understanding required for robust and reliable processes at an early stage. Consequently, issues such as oiling out, solvent and impurity entrapment, multimodal particle size distributions and previously unseen polymorph transitions have been all too common to first be seen upon process scale-up, often requiring significant quantities of additional time and investment to circumvent.
Get the white paper
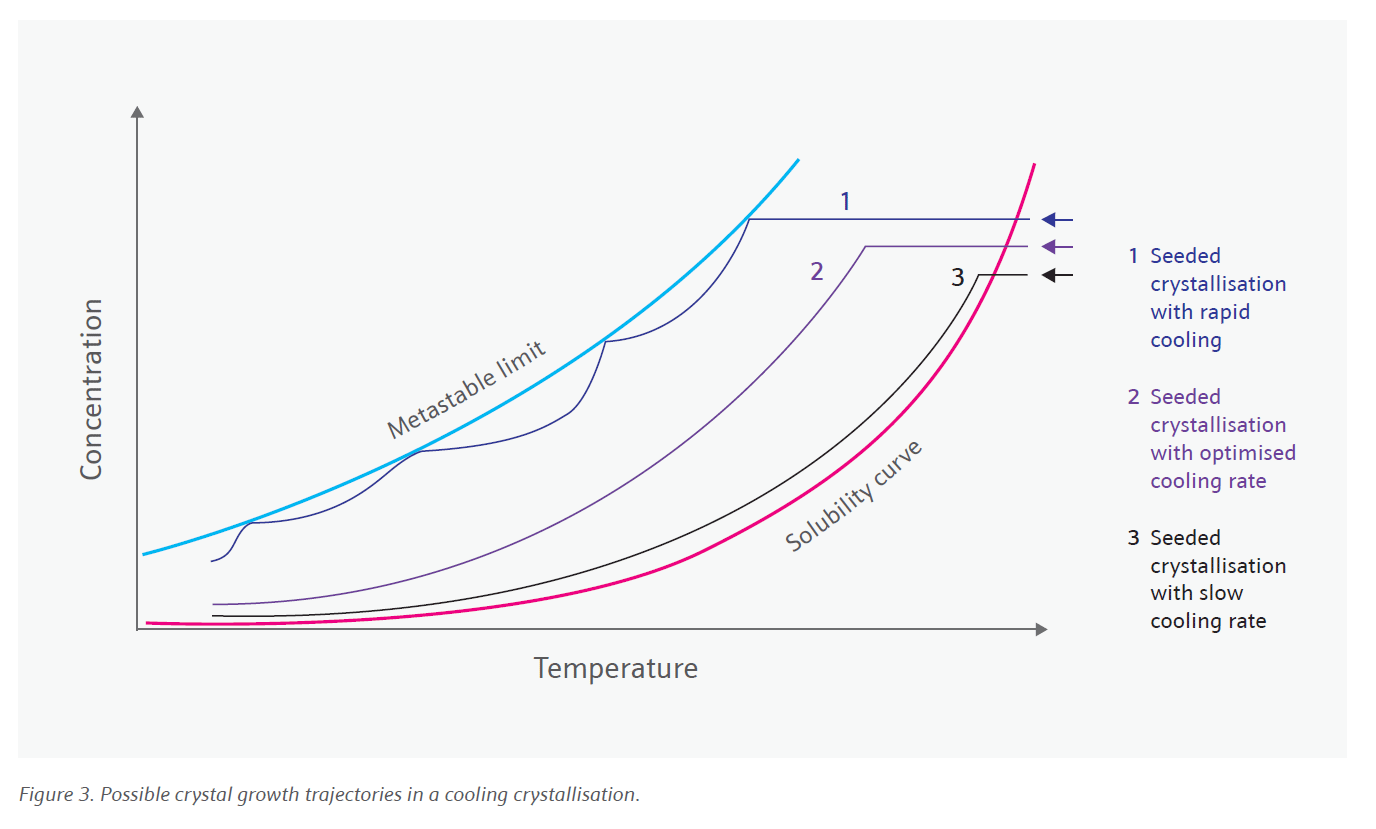
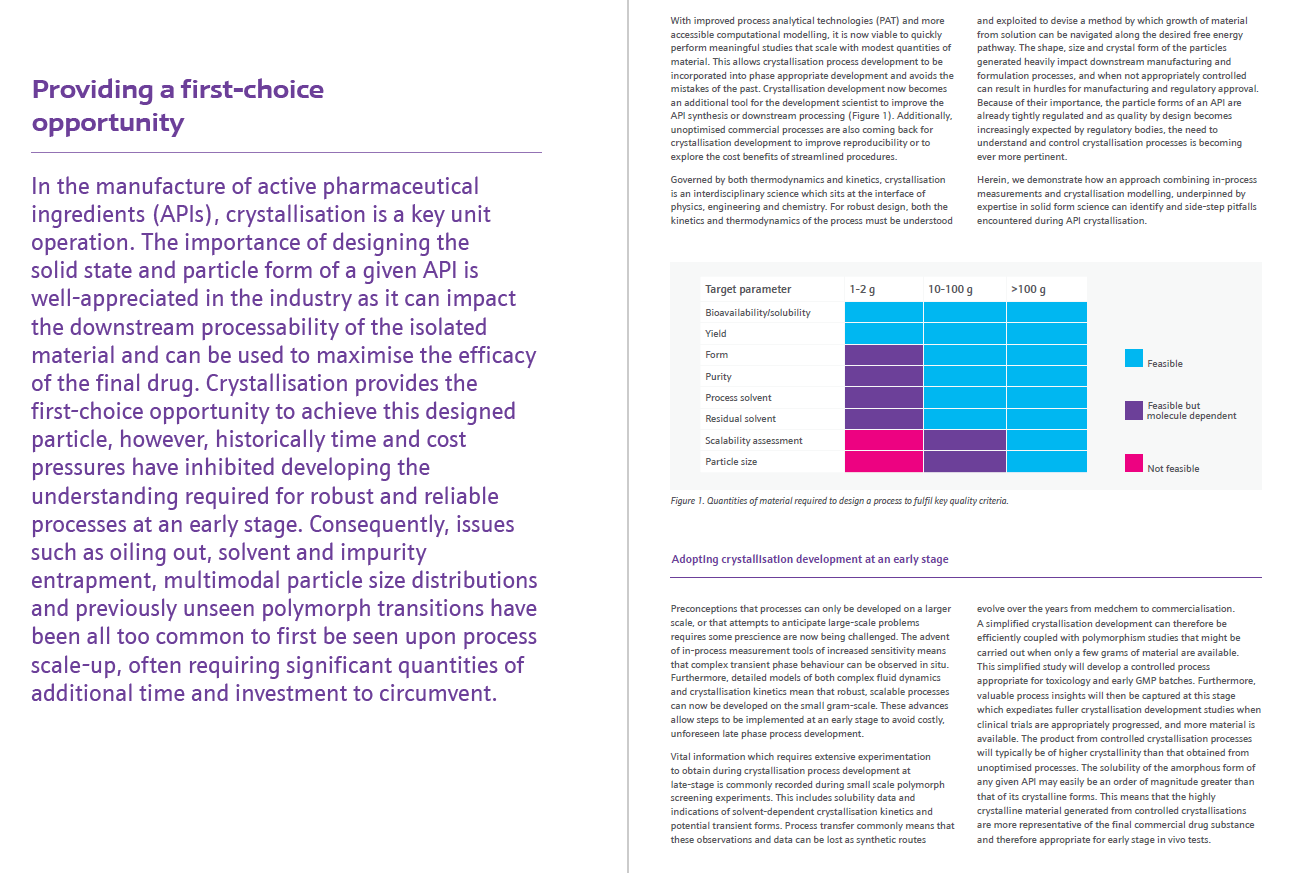
Solid state science has long been our forte at Johnson Matthey. In recent years we have been leveraging this understanding to develop highly controlled and robust crystallisation processes. Download the full white paper to read on and discover more
Read more
Markets
Read more on JM's solid form science expertise

Products and services
View all our pharmaceutical products