Early form screening strategies to accelerate candidate selection
26 August 2020
Failure to select a successful candidate can be expensive in terms of both time and money.
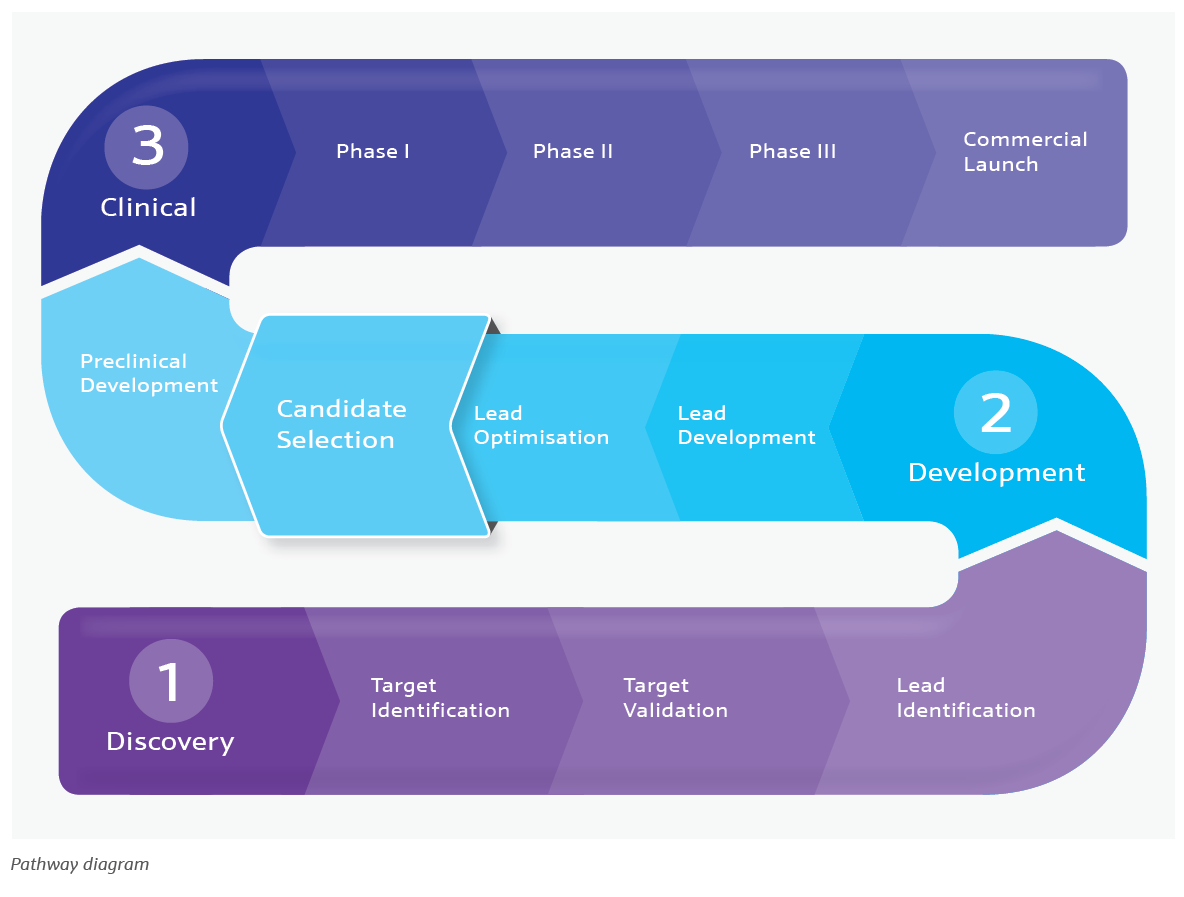
The development of a new drug often begins with a hypothesis. In particular, researchers are interested in understanding how an individual biological molecule, such as an enzyme or protein receptor, can be targeted to regulate its function and affect the disease process. The route to new medications follows a well-recognised drug discovery pathway (depicted below) which begins with the target identification and ends with the commercial launch of the drug product.
This route to drug discovery, development and manufacture is a notoriously lengthy process, and can often take over a decade from discovery to launch. Additionally, there can be many challenges and pitfalls that need to be addressed along the way. As the process moves forward, there comes a point at which the selection of a particular candidate and perhaps one or two backup candidates to go forward into the clinical phase is reached, this is termed clinical candidate selection.
Get the white paper
Download the full white paper to read on and discover more about early form screening strategies for pharmaceutical development and the importance of understanding an API’s solid form for formulation development.
Read more
Markets
Read more on JM's solid form science expertise

Products and services
View all our pharmaceutical products