Ene reductase enzyme technology
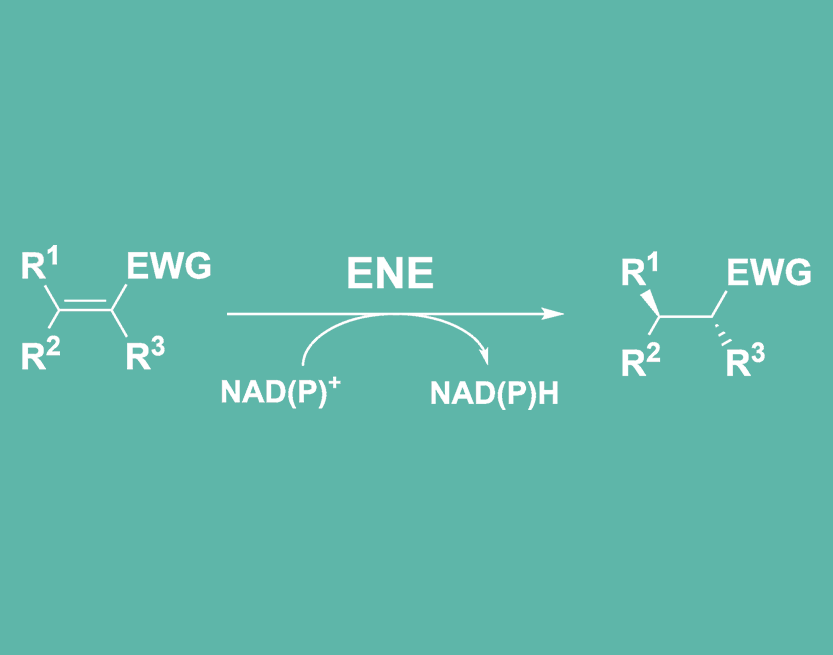
Ene reductase enzyme catalyse the reduction of C=C double bonds in presence of an electron withdrawing group (EWG). Examples of electron withdrawing groups are carbonyls (ketones and aldehydes) and nitro. In some specific instances, carboxylic acids, esters and nitriles can also act as activating groups.
Advantages of using ene reductases:
- Perform asymmetric trans hydrogenation of activated alkenes in aqueous media, and under mild conditions of temperature and pressure (1,2).
- Ene reductase can be used in cascade reactions with other enzymes, allowing for the synthesis of complex molecules in a single pot (3).
- Tuneable activity and selectivity by using enzyme engineering.
Ene reductase enzyme options
Get in touch
Send us a message to request a quote or ask our advice. We'd be happy to hear from you.
References:
1. Johnson Matthey Technol. Rev., 2016, 60, (4), 243–249
2. Johnson Matthey Technol. Rev., 2020, 64, (4), 529–53
3. J. Am. Chem. Soc. 2019, 141, 19208−19213
1. Johnson Matthey Technol. Rev., 2016, 60, (4), 243–249
2. Johnson Matthey Technol. Rev., 2020, 64, (4), 529–53
3. J. Am. Chem. Soc. 2019, 141, 19208−19213


