Platinum (Pt) is typically the most recognised PGM. It is used in a wide variety of applications and markets, from jewellery to automotive to glassmaking. Platinum has a high melting point and temperature stability, is highly resistant to corrosion and oxidation, and is an excellent catalyst.
Platinum uses
Platinum jewellery currently accounts for almost a quarter of platinum demand, but the largest use of platinum is in automotive catalytic converters. Platinum plays an important role as a catalyst to reduce exhaust emissions, required in both diesel-fuelled and gasoline-fuelled vehicles.
Platinum is also used as a catalyst to produce fuels and chemicals. Its ability to catalyse many processes is central to our global economy.
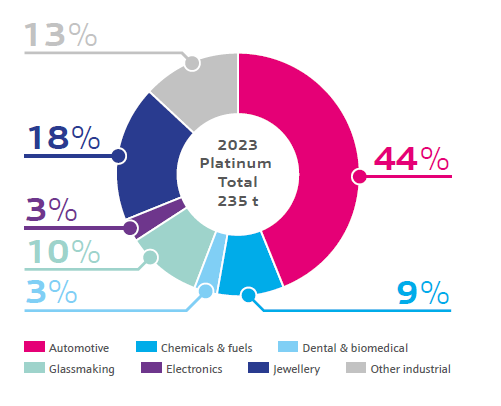

In medical applications, platinum is used in chemotherapy to treat a range of cancers as well as being used as an alloy to make dental materials and biomedical devices such as stents. In glassmaking, it is often alloyed with rhodium for fiberglass and high-quality flat glass used in TVs and phone screens.
Platinum is also essential for two major types of fuel cell technology: proton exchange membrane (PEM) fuel cells and phosphoric acid fuel cells (PAFC). As the world moves towards net zero carbon emissions, the growing use of fuel cell vehicles is expected to lead to significant platinum demand.




